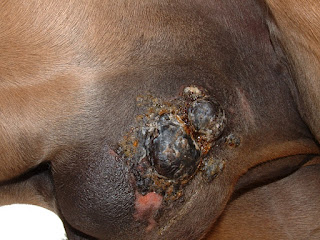
Sarcoids are the most common skin tumour in horses. Currently there is no universally effective treatment, and if treatment fails the sarcoids will often come back worse than they were in the first place. Although the disease is rarely life-threatening, many horses with sarcoids are euthanased because the condition is untreatable or because the horse is unsellable.
Interleukin (IL)-2 is one of the key cytokines (proteins involved in cell signalling). It stimulates cell mediated immunity, activating a range of T-cells. IL-2 is used in human medicine, including for treating cancerous conditions such as metastatic renal cell carcinomas and metastatic melanoma.
A canarypox virus vector, which has been genetically modified to contain a gene to produce IL-2, was used in the study. (The product is licenced for use in the treatment of fibrosarcoma in cats.) The technology is widely used in the production of vaccines for horses and other species, including some equine influenza and West Nile virus vaccines.
Corey Saba, of the University of Georgia College of Veterinary Medicine, Athens Georgia, and colleagues conducted a pilot study, which has been reported in the Journal of Veterinary Internal Medicine. The work was funded by Boehringer Ingelheim.
Fourteen otherwise healthy horses were included in the study. For a start, each sarcoid was measured and photographed, then injected with 1ml ALVAC-fIL2 divided between four or five different sites. Treatment was repeated 1,3 and 7 weeks later.
The research team monitored the response for at least a year after the final treatment. They found that size of the sarcoid reduced in 12 of the 14 horses in the study, with complete remission occurring in seven cases (50%). Partial remission occurred in a further five (35%)
An initial response was noted 34 – 406 days (median 89 days) after starting the treatment, and the best response occurred after 56 – 406 days (median 211 days).
Other than transient mild-moderate focal inflammation in two horses, the injections were well-tolerated.
The researchers conclude “our results provide evidence that ALVAC-fIL2 is a safe, cosmetic, and effective treatment for sarcoid tumors in horses. Furthermore, our results provide a basis for a larger, placebo-controlled study to better define the role of this treatment in sarcoid tumors in horses.”
For more details, see:
ALVAC-fIL2, a feline interleukin-2 immunomodulator, as a treatment for sarcoids in horses: A pilot study
Corey Saba, Randall Eggleston, Andrew Parks, John Peroni, Eric Sjoberg, Shelbe Rice, Jesse Tyma, Jarred Williams, Deborah Grosenbaugh, A. Timothy Leard
Journal of Veterinary Internal Medicine (2022);1–6.

No comments:
Post a Comment